Abstract
Background: Acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) represents a category of adverse-risk AML with poor overall survival and high rates of relapse. CPX-351 has shown promise in the treatment of therapy-related AML (t-AML) and AML-MRC. A recent phase III trial evaluated CPX-351 versus daunorubicin 60 mg/m2 + cytarabine (7+3) in newly diagnosed t-AML and AML-MRC and demonstrated superior responses and improvement in the median overall survival (OS). However, outcomes with CPX-351 versus 7+3 with daunorubicin 60 mg/m2 in the broader adverse-risk category and in the real-world setting remain unclear. The aim of this study was to characterize response and survival of both regimens in adverse-risk AML.
Patients & Methods: We analyzed 47 patients that underwent treatment for ELN 2017 adverse-risk AML from January 2016 to December 2021 at VCU Massey Cancer Center. Patients were divided into two cohorts: patients that received CPX-351 and 7+3 with daunorubicin 60 mg/m2. Baseline demographics were obtained, including age, performance status, and comorbidities, as well as disease characteristics, including molecular profiling either through PCR, NGS, or both at the time of induction, alongside cytogenetic risk, doses of induction regimens, response, and survival. Differences between groups were compared using an unpaired t-test. The event for calculating the OS through the Kaplan-Meier method was the date of death. Survival differences between groups were compared using the Gehan-Breslow-Wilcoxon test. Patients were censored at the date of last contact.
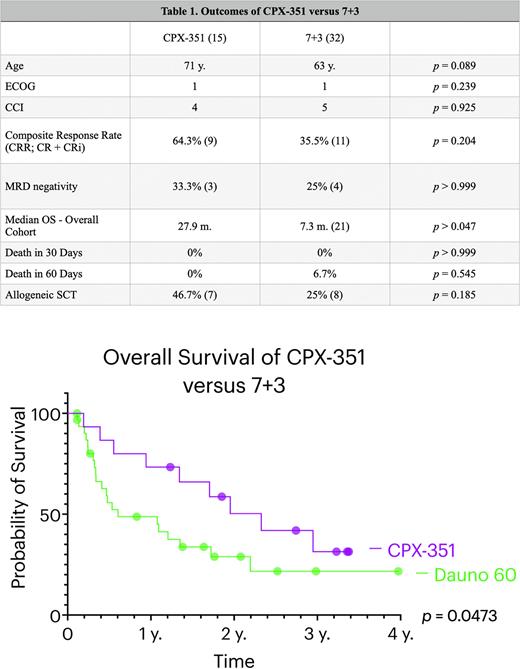
Results: There were 15 patients that underwent treatment with first-line CPX-351. The median age at diagnosis was 71 years, the median ECOG score was 1, and the median Charlson Comorbidity Index (CCI) score was 4. The composite response rate (CRR; CR + CRi) was 64.3% and 33.3% of evaluable patients were measurable residual disease (MRD) negative by PCR assays. The median OS was 27.9 months. There were no deaths in the first 60 days. Seven (46.7%) patients in the CPX-351 cohort proceeded to allogeneic stem cell transplant (SCT). In the 7+3 cohort, the median age was 63, median ECOG score was 1, and median CCI score was 5. There were no significant differences in age, ECOG score, or CCI score between the two cohorts. The CRR of the 7+3 cohort was 35.5% and 25% of those evaluable were MRD negative by PCR assays. The median OS was 7.3 months. No patients died within 30 days and 6.7% died within 60 days. Eight (25%) patients proceeded to SCT. The OS was then compared between cohorts, with CPX-351 demonstrating a significant improvement in survival compared to 7+3 (p = 0.047).
Conclusions: CPX-351 demonstrated a significant improvement in survival compared to 7+3 with daunorubicin 60 mg/m2 in our cohort of adverse-risk AML. Additionally, composite response rates appeared to be 1.8 times higher in the CPX-351 cohort with fewer patient deaths and an increase in receipt of SCT by 21.7%. These findings support the use of CPX-351 in patients with adverse-risk AML that are candidates for intensive induction.
Disclosures
Maher:BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal